BACKGROUND:
Hairy cell leukemia (HCL) is a rare indolent B-cell lymphoproliferative disorder. The BRAF V600E mutation was shown to be the underlying genetic driver for the vast majority of classical HCL (HCLc) cases and treatment with the BRAF inhibitor (BRAFi), vemurafenib, has proven extremely successful.
Dabrafenib is an oral BRAFi used in melanoma, often in conjunction with trametinib, a MEK inhibitor. Data exists on the use of dabrafenib combined with trametinib in HCLc but there is no efficacy or safety data on either single agent dabrafenib or dabrafenib combined with rituximab. We present a small series of 7 patients treated with either single agent dabrafenib or combination dabrafenib and rituximab.
METHODS:
Since 2018 dabrafenib was accessible via a Novartis managed access programme for BRAF V600E mutated HCLc patients who had no alternative treatment options.
Six patients received dabrafenib, 2 as single agent due to recent prior ineffective rituximab therapy or severe rituximab infusion reaction and the remaining 4 patients with dabrafenib and rituximab. Dabrafenib was given at 150mg twice daily on a continuous 28 day cycle. Rituximab 375mg/m2 IV was given once every 2 weeks for a total of 8 doses. As there was no availability for re-treatment on cessation of therapy and no other suitable/accessible treatment options, patients with ongoing response and good tolerance were continued on indefinite therapy.
One additional patient was treated with frontline dabrafenib and rituximab due to various comorbidities including end stage renal failure limiting other treatment options.
RESULTS:
Mean age on commencing dabrafenib was 73 (52-87) and in the relapsed patients the median number of prior lines of therapy was 5 (3-13). Four patients had undergone prior splenectomy. Two patients had received prior BRAFi therapy with vemurafenib and 3 had received prior Moxetumomab.
Patients received a median of 8 cycles of dabrafenib. 4 patients received all 8 cycles of rituximab with 1 discontinuing rituximab due to adverse drug reaction.
Adverse drug reactions to dabrafenib were mostly grade 1-2. The most common reaction was benign keratotic skin lesions occurring in 5/7 patients. Their onset was typically after 4 weeks of treatment, persisting whilst therapy continued. Other notable adverse drug reactions were hair thinning and palmar-plantar erythrodysesthesia both occurring in 3/7 patients. Hair thinning did seem to improve with decreasing the dosage and resolve on cessation of the drug. Palmar-plantar erythrodysesthesia was managed with dose reduction and non-steroidal anti-inflammatory drugs.
Response assessment criteria used were from the 2017 hairy cell leukemia foundation consensus guidelines for the diagnosis and management of patients with HCLc.
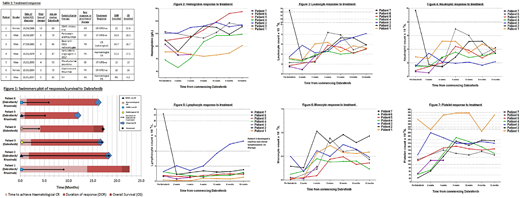
All patients responded to therapy. Hematological response was rapid, most patients achieving hematological CR after 2-3 cycles. Five patients achieved a CR, 2 were MRD+ve, 2 were MRD-ve and 1 was a radiological CR. The remaining 2 patients achieved a hematological response, 1 partial and 1 complete. Six patients had sufficient follow up for assessment with 2/6 having relapsed and 4/6 remaining in remission. Median follow up was 17 months, median DOR and median OS were not reached.
The 2 patients in our cohort (patients 1 and 3) receiving prior vemurafenib each received 6 cycles, 1 achieving CR lasting 3 months, the other achieving PR lasting 11 months. Both responded rapidly to dabrafenib, one treated with dabrafenib and rituximab achieved an MRD+ve CR with DOR 21 months. The other received single agent dabrafenib and achieved a hematological response with DOR 15.2 months.
Treatment response and survival are shown in table 1 and figure 1. Figures 2-7 show the hematological response at time points within the first year of therapy.
DISCUSSION:
In this small cohort we have shown that dabrafenib as a single agent or combined with rituximab is a safe and effective therapy in BRAF V600E mutated HCLc, even in those patients with prior time limited duration BRAFi therapy. There was no hematological toxicity noted. The main adverse drug effect was development of skin lesions however none were malignant. The only malignant lesion noted pre dated the commencement of dabrafenib. Dermatological surveillance is therefore recommended. Hematological response was rapid with significant improvement seen as early as 4 weeks from commencing therapy.
El-Sharkawi:Janssen:Honoraria, Membership on an entity's Board of Directors or advisory committees;Roche:Other: Conference fees.
Use of BRAF inhibitor Dabrafenib in BRAF V600E mutation positive classical Hairy Cell Leukemia.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal